The human immune system is a sophisticated network designed to detect and eliminate pathogens, but some viruses, like HIV, have evolved ingenious mechanisms to exploit its defenses. One of HIV’s strategies involves manipulating Toll-like receptors (TLRs), critical components of the innate immune system that detect pathogen-associated molecular patterns (PAMPs) and initiate immune responses.
This article explores how the HIV virus interacts with TLRs, particularly on macrophages, to facilitate its replication. While macrophages play an essential role in immune defense, HIV cleverly activates these cells to create an environment conducive to its lifecycle. Additionally, we will examine the interplay between innate and adaptive immunity, highlighting the role of Langerhans cells and soluble factors in bridging these systems. By understanding these interactions, we gain insight into how the immune system responds to threats and how HIV subverts these defenses to persist and spread.
How HIV Exploits Macrophages via TLRs
HIV is a virus that has evolved sophisticated mechanisms to exploit the immune system, particularly through its interactions with Toll-like receptors (TLRs) on immune cells like macrophages. CD4 T cells are the primary targets of HIV, but macrophages also play a crucial role in the virus’s lifecycle. Surprisingly, HIV encodes ligands (soluble molecules) that bind to TLRs on macrophages, activating them to a pro-inflammatory state. At first glance, this seems counterproductive—wouldn’t an activated macrophage attack and eliminate the virus?
This is not the case. Macrophages in a quiescent (inactive) state produce minimal amounts of proteins. An inactive macrophage is a poor environment for viral replication because HIV requires the production of host proteins for its lifecycle. By activating macrophages through TLR signaling, HIV turns the macrophage into a highly active protein-producing factory, thereby creating the conditions necessary for viral replication. HIV employs a similar strategy with T cells. Quiescent T cells are not conducive to viral replication, but once activated, they provide the resources HIV needs to multiply and spread.

The Link Between Innate and Adaptive Immunity: Langerhans Cells
A critical step in immune defense is bridging the innate immune system with the adaptive response. This connection is achieved through antigen presentation by specialized cells like Langerhans cells, a type of immature dendritic cell found in the skin and mucosal tissues.
When pathogens, such as bacteria, breach the skin, Langerhans cells recognize them using pattern-recognition receptors like CD14 and TLRs. For instance, when a bacterium binds to CD14, the activated CD14 receptor recruits and stimulates TLR4, which then activates the Langerhans cell. The activated Langerhans cell will then migrate from the epidermis to the lymph nodes, carrying digested antigens from the invading pathogen.
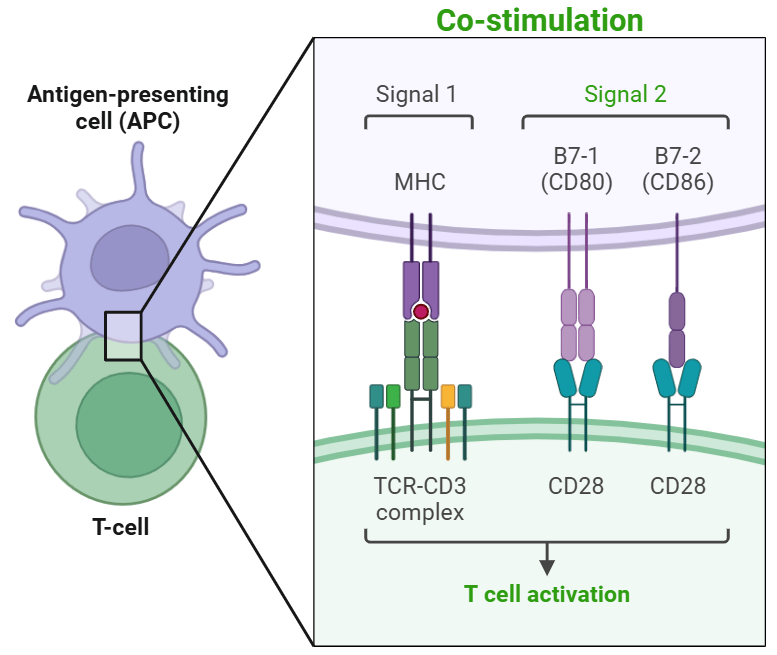
Once in the lymph nodes, these cells mature into highly effective antigen-presenting cells (APCs). They express major histocompatibility complex (MHC) molecules and present the bacterial antigens to T and B lymphocytes, activating the adaptive immune system to combat the infection by targeting the specific bacterial antigen that was presented.
The Role of Soluble Factors in Pathogen Recognition
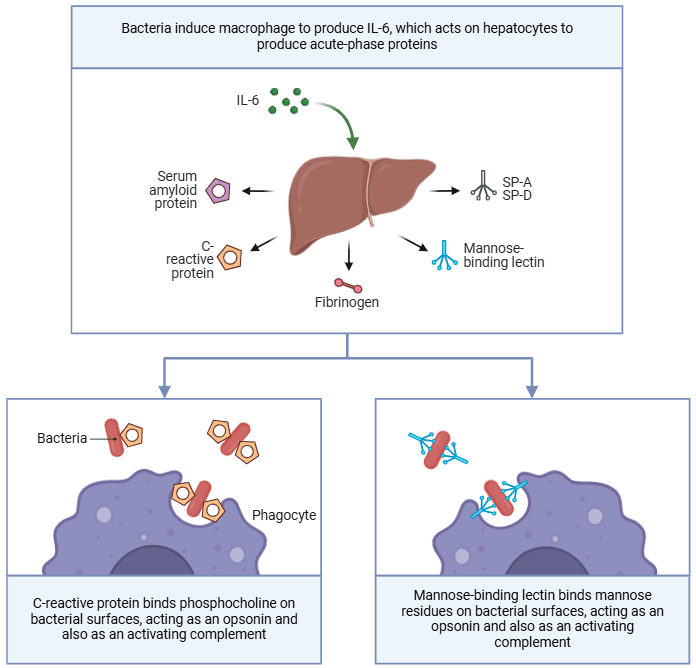
In addition to cell-surface receptors, the innate immune system utilizes soluble factors that recognize pathogen-associated molecular patterns (PAMPs). These factors function similarly to the antibodies of the adaptive immune system but are less specific and act more rapidly. Examples of these soluble factors include:
- Mannose-Binding Lectin (MBL):
- Like the mannose receptors on macrophages, soluble MBL binds to mannose motifs on pathogens, aiding in their identification and elimination by the host’s immune system.
- C-Reactive Protein (CRP):
- CRP binds to phosphocholine on bacterial surfaces, tagging the bacteria for phagocytosis.
- This process enhances the efficiency of macrophages, linking soluble factors to pathogen surfaces and improving immune clearance.
- CRP levels in the blood serve as a biomarker for bacterial infections, providing clinicians with a tool to monitor the presence of infection.
These soluble factors reflect the innate immune system’s ability to mimic certain functions of the adaptive immune system, offering a rapid and versatile response to infections.
The Parallels Between Innate and Adaptive Immunity
The innate immune system, like the adaptive immune system, has mechanisms for recognizing antigens and pathogen motifs. While adaptive immunity relies on highly specific antigen receptors on T and B cells, innate immunity uses pattern-recognition receptors (e.g., TLRs, mannose receptors) and soluble factors (e.g., CRP, MBL). This redundancy ensures robust protection against a wide range of pathogens, even in the absence of a fully activated adaptive immune response.
Conclusion
The interplay between HIV and the immune system highlights the complexity of viral immune evasion strategies. By leveraging TLR signaling, HIV activates macrophages to create an environment conducive to its replication. At the same time, the innate immune system plays a crucial role in bridging to the adaptive response through cells like Langerhans cells and soluble factors like CRP and MBL. This dual approach, combining cellular and soluble components, underscores the sophistication of innate immunity in recognizing and responding to infections, even in the face of challenges like HIV.
Having covered the innate immune system in great depth, in our next Immunology blog, we will discuss how T cells recognize specific non-self antigens!
