The adaptive immune system is incredibly robust, recognizing and eliminating a variety of different pathogenic contexts. The driving force behind the adaptive immune system are B and T cells. These cells have the same generic function – eliminate specific foreign invaders via antigen recognition. Remember from our previous Immunology blog that antigens are anything that the immune system can recognize, which is not limited to chemicals, pollutants, and pathogens. However, despite the same goal of recognizing and eliminating foreign antigens, B & T cells go about this process differently. Essentially, these 2 pivotal cell types recognize different antigenic contexts.
Let’s first discuss T cells! T cells have cell surface receptors that that recognize primary structures via ligand-receptor binding. The cell surface receptors are the antigen recognition molecules of T cells. A primary structure refers to the amino acid sequence of a protein, which are a type of antigen. These foreign proteins are broken down and digested into its simplest amino acid sequence, which is then presented in as a major histocompatibility complex molecule, which is presented as a cell surface protein on antigen presenting cells, such as macrophages and dendritic cells, which are members of the fast acting innate immune system. A key component of the immune system’s function is the communication between its innate and adaptive cell types, evident in T cell antigen recognition. On the other hand, B cells use an immunoglobulin molecule, more commonly known as an antibody, to recognize the soluble 3D structures of the native antigen molecules. Unlike T cells, the antigen recognition molecules of B cells are the antibody molecules that it secretes.
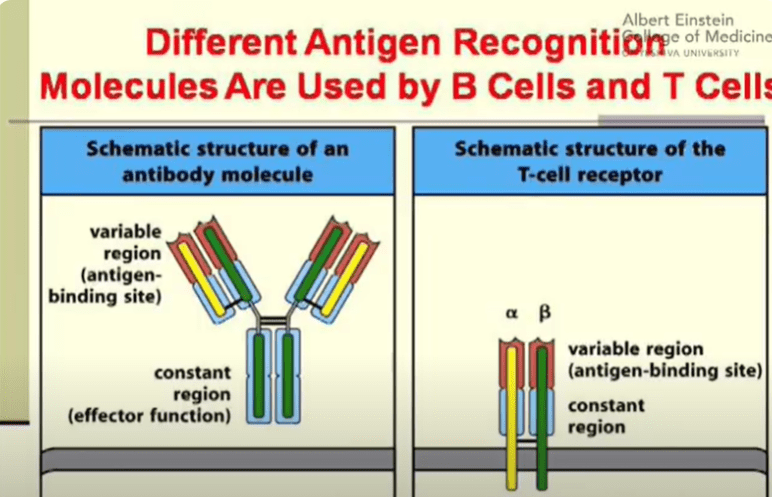
Let’s now break down the differences in the structure between T cell surface receptors and B cell antibodies. The most common T cell surface receptors are composed of 2 polypeptide chains – the alpha chain and the beta chain, which are held together via a disulfide bond. A disulfide bond (-S-S-) is a crucial component of tertiary protein structure formed by the terminal thiol (-SH) groups of 2 cysteine amino acids. These bridges are excellent for maintaining and holding protein structure in place. Each polypeptide chain is composed of a variable domain and a constant domain. The variable domains are the sites where the antigen binds to. In contrast, the antibodies secreted by B cells have a slightly different structure. Antibodies are composed of 2 heavy chains and 2 light chains, which are held together by disulfide bonds. Each light chain is composed of 1 variable and 1 constant domain while each heavy chain is composed of 1 variable and 3 constant domains. Just like in T cell receptors, the variable domains are the antigen binding sites. The constant region in antibodies determine the effector function and protein binding of antibodies.
Now that we’ve discussed the differences in antigen binding molecules of T and B cells, let’s dive into the different roles of T cells. T cells can function as Cytotoxic T Cells or Helper T Cells. Cytotoxic T cells, also known as pessimistic cells, are activated when your body’s cells are infected by a virus. The infected cells then present peptides from that virus on their cell surface, activating the cytotoxic T cell to kill the infected cell. Cytotoxic T cells are known as “pessimistic cells” because once a cell has been infected, if is considered to be “unsavable” and therefore, must be eliminated. On the other hand, helper cells are involved in activating macrophages in the immune response, increasing their efficiency as immune cells, enabling them to clear the infections. Unlike cytotoxic T cells, helper T cells are known as “optimistic cells,” as even if a cell have been infected, the immune system still works to save that cell and eliminate only the infection.
In our next Immunology blog, we will discuss the intricacies involved in initiating an immune response! See you next time!